-
Call Now
1800-102-2727
Types of Oxide Formed - Classification of Oxides, Nature of Oxides, Practice Problems and FAQs
You know that the quality of the air we breathe is deteriorating due to the air pollution but do you know what is the main reason for the air pollution? Air pollution refers to biological, chemical and physical changes in the air which are mainly caused due to the oxides of various elements like nitrogen, sulphur and carbon produced by industries and vehicle exhaust. Like carbon monoxide which is produced due to incomplete burning of the fuel. Do you know carbon monoxide when inhaled in a significantly high amount can cause the death of an individual? But what is the reason behind it? So, when you inhale carbon monoxide it combines with the haemoglobin present in the blood vessel and forms a stable complex called carboxyhemoglobin complex. Due to the formation of this complex, it doesn't allow the transfer of oxygen in our body and eventually causes death. Let’s have a look at the types of oxides that can be formed from the elements, and their classification and discuss the nature of oxides.
Table of content
- Classification of Oxides
- Practice Problems
- Frequently Asked Questions-FAQs
Classification of Oxides
One of the most important components in the atmosphere is oxygen. This element is necessary for the existence of all living species on the earth in its gaseous diatomic state. The atomic number of oxygen is 8 and it is found in group 16, period 2 of the current periodic table. It is the first of the chalcogen family members. Oxygen makes up 21% of the earth's atmosphere, and metals in their oxide forms make up more than half of the earth's crust.
When oxygen is linked with the less electronegative element it results in the formation of the oxides. For example
In general, oxides are divided into two categories:
- Metallic oxides
- Non-metallic oxides
Metallic oxides:
Metallic oxides are the type of oxides which are produced when metal reacts with oxygen. For example,
When sodium metal combines with oxygen, sodium oxide is produced, which is a metallic oxide.
The elements in the s-block are metals; they usually react with oxygen to form metallic oxides.
Non-metallic oxides:
When non-metal combines with oxygen, non-metallic oxides are produced. For example,
When carbon is burned in the presence of excess air, it produces carbon dioxide.
Sulphur dioxide gas is produced when sulphur is burned in the air.
p-block elements, in general, react with oxygen to form non-metallic oxides.
On the basis of nature, oxides are classified into four different types:
- Acidic oxides: When dissolved in water, these oxides produce an acidic solution. At 25 °C, the pH of the resulting oxide solution is less than 7. For example,
Nitric acid is formed when nitrogen pentaoxide is combined with water.
Sulphur trioxide forms sulphuric acid when it combines with water.
Some other examples of acidic oxides are etc.
- Basic oxides: When these oxides are dissolved in water, they produce basic solutions. At 25 °C, the pH of the resulting oxide solution is more than 7. For example,
When sodium oxide reacts with water it results in the formation of a basic sodium hydroxide solution.
When thallium oxide which is a basic oxide dissolved in water it results in the formation of a basic thallium hydroxide solution.
Some other examples of basic oxides are MgO, CaO etc.
- Amphoteric oxides: These are the oxides that can react with both acids and bases to produce salt. When it reacts with an acidic solution, it behaves as a basic oxide, producing salt, and vice versa. For example, etc.
When aluminium oxide reacts with sodium hydroxide base, it results in the formation of sodium aluminate and water.
When aluminium oxide reacts with hydrochloric acid, it results in the formation of aluminium chloride salt and water.
- Neutral oxides: These are the types of oxide which are neither basic nor acidic in nature. At 25 °C, the pH of the neutral oxide solution is 7. For example,
Classification of oxides on the basis of the oxidation state of the oxygen atom in the oxide:
- Normal oxides: These are the type of oxides in which the oxidation state of oxygen is -2. For example Na2O, MgO, CaO, H2O etc.
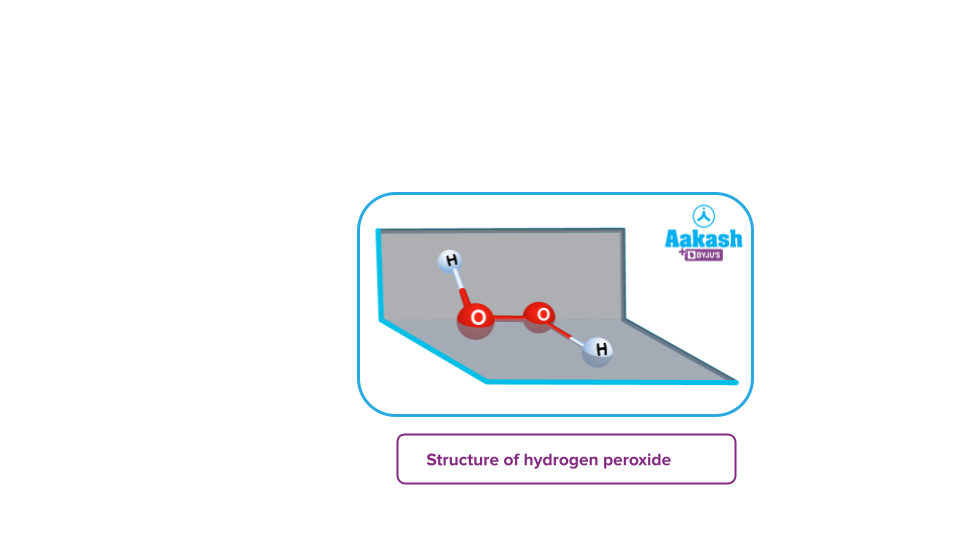
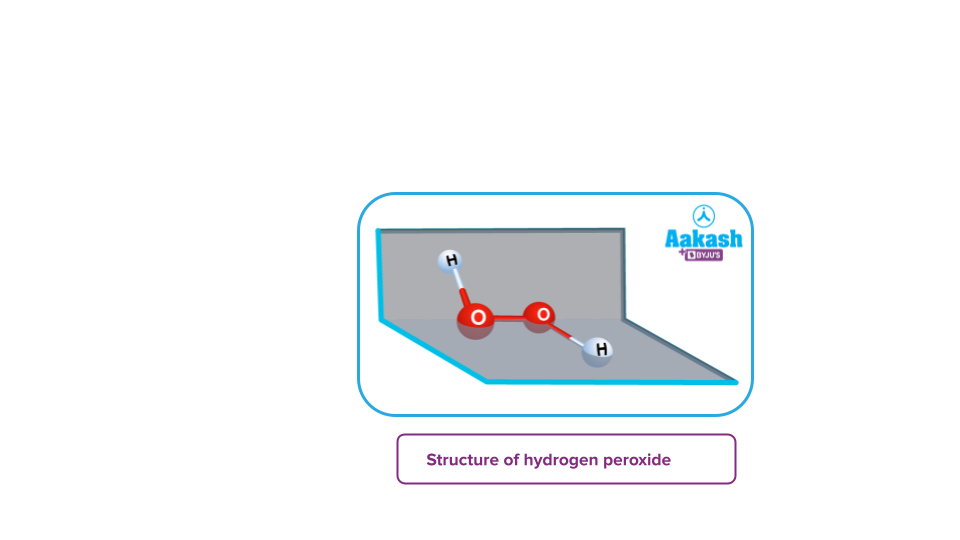
- Peroxides: These are the type of oxides in which the oxidation state of the oxygen atom corresponds to -1. For example- H2O2, BaO2, Na2O2 etc.
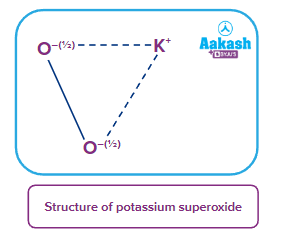
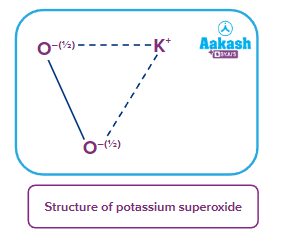
- Superoxides: These are the type of oxides in which the average oxidation state of oxygen atom is For example: KO2, NaO2, RbO2 etc.
Some other types of oxides:
- Mixed oxides: These are the type of oxides which are formed by mixing two normal oxides. For example- Fe3O4 which contains two different types of oxide, Iron (II) oxide (FeO) and Iron(III) oxide (Fe2O3). similarly Pb3O4 which contains two different oxides, lead monoxide (PbO) and lead dioxide (PbO2).
- Sesquioxides: These are the type of oxides which contain 2 atoms of other element and 3 atoms of oxygen. The general formula of representation of the sesquioxide includes M2O3 where ‘M’ represents the element which forms oxides. For example, Boric oxide ( B2O3), Aluminium oxide (Al2O3) etc.
- Sub oxides: These are the type of oxides which are formed when the element forming an oxide is in excess as compared to the oxygen atom. For example carbon suboxide (C3O2), boron suboxide (B6O), Nitrous oxide (N2O) etc.
Practice Problems
Q1. Select the correct formula for the oxides among the following which are neutral in nature.(H2O, CO, CO2, N2O5)
- H2O and CO
- CO2 and CO
- N2O5 and H2O
- CO2 and N2O5
Answer: (A)
Solution: As we know, the neutral oxide is the type of oxide which is neither basic nor acidic in nature. At 25 °C, the pH of the neutral oxide solution is 7. For example, NO, CO, N2O, H2O. Whereas CO2 are N2O5 acidic oxides, when dissolved in water form an acidic solution.
Q2. Which among the following list of compounds contains the peroxide form of oxygen?
- H2O2
- BaO2
- KO2
- Both A and B
Answer: (D)
Solution: Peroxide is the type of oxide in which the oxidation state of the oxygen atom corresponds to -1. For example- H2O2, BaO2, Na2O2 etc. Whereas KO2 is an example of superoxide in which the average oxidation state of the oxygen atom is .
Q3. The average oxidation state of the oxygen atom in superoxide form is:
- 1
- -2
- 0
Answer: (B)
Solution: Superoxide is the type of oxide in which the average oxidation state of oxygen atom is -(). For example: KO2, NaO2, RbO2 etc. Whereas the compound in which the oxidation state of oxygen is -2 is generally called as simple oxide or normal oxide and (-1) oxidation state corresponds to the peroxide compound.
Q4. Which of the following options is correct with respect to the oxidation state of oxygen atoms in different compounds.
- Average oxidation state of oxygen in oxide form is -1
- Average oxidation state of oxygen in peroxide form is -2
- Average oxidation state of oxygen in superoxide form is
- Oxidation state of oxygen atom in its molecular form is -2
Answer:
Solution: Oxidation state of oxygen in oxide form is -2, in superoxide form average oxidation state corresponds to (), the oxidation state of peroxide is (-1) respectively. In the case of the molecular form of the oxygen, the oxidation state of each oxygen atom corresponds to 0.
Frequently Asked Questions - FAQs
Q1. What is the difference between peroxide and superoxide?
Answer: The main difference between peroxide and superoxide includes:
|
Peroxide |
Superoxide |
|
It is the type of oxide in which the oxidation state of the oxygen atom corresponds to -1. |
It is the type of oxide in which the average oxidation state of the oxygen atom is . |
|
Example: H2O2, BaO2, Na2O2 etc. |
Example: KO2, NaO2, RbO2 etc. |
|
|
|
Q2. What are the uses of the oxides?
Answer: Some important uses of the oxides are:
- Calcium oxide is used as a dehydrating agent and absorbs moisture.
- Low reactive metal oxides like (HgO, Ag2O) help in the extraction of metal when decomposed at at a high temperature.
- Metal oxide like Al2O3 helps in the extraction of aluminium element when electrolysed.
- Oxides of sulphur help in the preparation of sulphuric acid by contact process
- Oxide of nitrogen help in the manufacture of nitric acid through Birkeland and Edye method or Ostwald’s method.
Q3. What is the trend of the nature of oxides of elements across the period in the periodic table?
Answer: As we know that on the basis of nature, elements can form four different types of oxides namely- acidic oxide, basic oxide, amphoteric oxide and neutral oxide. Metals have a general tendency to form basic oxides and non-metals have a general tendency to form acidic oxides. As we move from left to right in a period, the metallic character of an element decreases which results in a decrease in the basic nature of oxide. For example,
In the 2nd period: Li2O is basic in nature, BeO is an amphoteric oxide and (CO2, N2O5) are acidic oxides.
Q4. What is the trend of the nature of oxides of elements moving down the group in the periodic table?
Answer: As we know that on the basis of nature, elements can form four different types of oxide namely- acidic oxide, basic oxide, amphoteric oxide and neutral oxide. Metals have a general tendency to form basic oxides and non-metals have a general tendency to form acidic oxides. As we move down the group in a periodic table, the metallic character of an element increases due to the increase in the number of shells and therefore the acidic nature of the oxide generally decreases.
For example, the nature of oxides of group-14 is listed below:
|
Formula of oxide |
Nature |
|
CO2 |
Acidic |
|
SiO2 |
Acidic |
|
GeO2 |
Amphoteric |
|
SnO, SnO2 |
Amphoteric |
|
PbO, PbO2 |
Amphoteric |